Maggiore morbilità dei MSM nelle malattie sessualmente trasmissibili
(MSM = Men having Sex with Men; MST = Malattie Sessualmente Trasmissibili)
Poche settimane fa il dottor Antonio Cristaudo, ha riassunto gli esiti del Congresso ADOI 2017, il 56mo (Associazione Dermatologi Ospedalieri Italiani), di cui era presidente -.”In Europa, dalla metà degli anni ’90 alcune MST hanno trovato ‘terreno fertile’ per espandersi dopo un decennio di declino dei trend epidemiologici, soprattutto nelle grandi metropoli e in alcuni gruppi di popolazione maggiormente a rischio, ad esempio i maschi omosessuali.
La sifilide, ad esempio, in Italia è cresciuta del 400% dal 2000 e se i casi da virus HIV sono stabili tra i giovani, un picco di nuove infezioni si registra tra gli over 50. La gonorrea, invece, ha visto quasi raddoppiare i casi in Europa tra il 2008 al 2013.
Questi dati sono confermati anche fuori dall’Europa. Secondo l’Organizzazione Mondiale della Sanità (OMS) ogni anno l’impatto di quattro MST, tra le più diffuse, corrisponde a 498,9 milioni di nuovi casi. Questo vuol dire che nel mondo oltre un milione e mezzo di persone ogni giorno si ammala per una MST. In Italia, secondo i dati dell’Istituto Superiore di Sanità, negli ultimi anni i casi di MST sono sempre aumentati, passando dai circa 3500 del 2006 ai circa 6500 del 2013.
http://www.ifo.it/de/index/news/Ottobre-2017/56%C2%B0-Congresso-ADOI.html
Singolare è anche il caso del Linfogranuloma venereo, nome che identifica i particolari sierotipi L1 L2 e L3 di Chlamidia prima confinate in India, Est Asiatico e Sud America e dal 2003 progressivamente in aumento anche in Europa soprattutto nella comunità più a rischio di maschi omosessuali. Un contagio che si accompagna con un picco di infezioni di Epatite C perché si è ipotizzato che le lesioni della mucosa rettale favoriscano anche da diffusione del virus HCV e di quelle da HIV .
Sino a pochi anni fa si trattava di una patologia rara mentre di recente l’ESSTI European Surveillance of Sexually Transmitted Infections l’ha definita una ‘vera e propria epidemia’. I casi di cervicite da Chlamydia trachomatis hanno mostrato una modica riduzione fino al 2000 e un successivo incremento, con un aumento più che doppio dei casi rispetto a quelli segnalati nel 2000.
Il lavoro è questo: http://sti.bmj.com/content/80/4/255
Riassume tutto ed è corredato da una ricca bibliografia. È alla fine come Allegato A
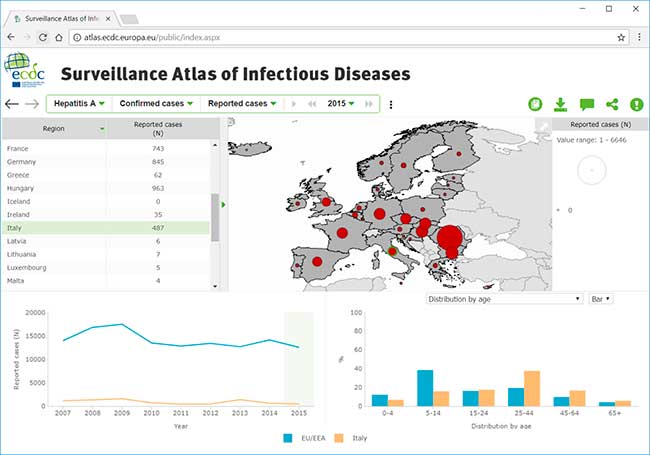
In questo file la documentazione dei dati epidemiologici della malattie infettive in Europa, dove si evince che le malattie sessualmente trasmissibili colpiscono soprattutto MSM: http://atlas.ecdc.europa.eu/public/index.aspx
Nelle pagine seguenti una rassegna di lavori scientifici nel periodo considerato, dagli anni ’90 ad oggi, che abbracciano anche regioni fuori dall’Europa, che confermano le affermazioni dell’aumento delle malattie sessualmente trasmissibili e di come la categoria più colpita sia quella degli MSM.
HIV:
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/HIV-AIDS-surveillance-Europe-2015.pdf
- https://www.cdc.gov/hiv/group/msm/index.html
- https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2015-vol-27.pdf
- https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- http://www.eurosurveillance.org/upload/site-assets/imgs/Special_Edition_2_2015_HIV.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/HIV%20and%20men%20who%20have%20sex%20with%20men.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/Dublin-EB-HIV%20and%20laws%20and%20policies%202017%20final.pdf
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/hiv-sti-prevention-among-men-who-have-sex-with-men-guidance.pdf
Epatite:
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/13-12-2016-RRA-Hepatitis%20A-United%20Kingdom.pdf
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/16-02-2017-RRA%20UPDATE%201-Hepatitis%20A-United%20Kingdom.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/RRA-19-May-2017_UPDATE_2-HepatitisA-in-mostly-MSM.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/RRA%20hep%20A%20outbreak%20EU%20EEA%20in%20MSM%20third%20update%2028%20June%202017_0.pdf
Sifilide:
Linfogranuloma venereo:
- https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-Lymphogranuloma-venereum.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/24655220
- https://www.ncbi.nlm.nih.gov/pubmed/24642056
- https://www.ncbi.nlm.nih.gov/pubmed/26675210
- https://www.ncbi.nlm.nih.gov/pubmed/29022544
Clamidia:
- https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-chlamydia.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/29066627
Gonorrea:
- https://ecdc.europa.eu/sites/portal/files/documents/AER_for_2015-gonorrhoea.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/29021080
- https://www.ncbi.nlm.nih.gov/pubmed/28943174
- https://www.ncbi.nlm.nih.gov/pubmed/28942419
Papilloma:
- https://www.ncbi.nlm.nih.gov/pubmed/29141014
- https://www.ncbi.nlm.nih.gov/pubmed/28910359
- https://www.ncbi.nlm.nih.gov/pubmed/28906006
Tumore anale:
- http://www.startoncology.net/area-professionale/tumore-della-regione-anale/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4788521/
- https://www.ncbi.nlm.nih.gov/pubmed/27585942
- https://www.ncbi.nlm.nih.gov/pubmed/24072194
- https://www.cdc.gov/std/hpv/hpvandmen-fact-sheet-february-2012.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/27789574
Danni meccanici dell’ano:
Più in dettaglio alcune patologie:
AIDS
Gli omosessuali hanno un rischio di contrarre l’HIV 19 volte più alto del resto della popolazione, e in questo gruppo i contagi stanno ”esplodendo”. Lo affermano le nuove linee guida dell’Oms, che per la prima volta consigliano ai gay di assumere i farmaci antiretrovirali come forma di prevenzione. Constatiamo una esplosione dell’epidemia in questo gruppo a rischio – ha affermato Gottfried Hirnschall, che dirige il dipartimento Hiv dell’Oms – soprattutto per un abbassamento della guardia dal punto di vista della prevenzione». Lo scorso maggio le autorità sanitarie statunitensi avevano consigliato i farmaci a tutti i gruppi a rischio, sulla base di studi che indicano che una pillola al giorno unita al preservativo abbassa il rischio del 25%. «Se gli omosessuali seguissero questa profilassi – sottolinea il comunicato dell’Oms – si potrebbero evitare un milione di nuovi contagi in dieci anni».
Europa
Al Centro europeo per la prevenzione e il controllo delle malattie (ECDC), agenzia indipendente dell’Unione europea, affermano come nel 2015 nei paesi dell’Ue e dell’Area Economica Allargata, vi siano state 30mila nuove notifiche di casi. Tra le nuove diagnosi, il 42% riguarda uomini che hanno fatto sesso con uomini, il 32% i rapporti eterosessuali e il 4% l’uso di siringhe infette, dato che gli uomini che fanno sesso con altri uomini sono una percentuale che oscilla tra il 2 e il 4% secondo le statistiche si deduce che sono il gruppo enormemente più colpito.
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/HIV-AIDS-surveillance-Europe-2015.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/Dublin-EB-HIV%20and%20laws%20and%20policies%202017%20final.pdf
USA
- https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- https://www.cdc.gov/hiv/group/msm/index.html
- https://www.cdc.gov/hiv/pdf/group/msm/cdc-hiv-msm.pdf
- Diagnoses of HIV infection in the United States and dependent areas, 2015. HIV Surveillance Report 2016;27.
- High-impact HIV prevention: CDC’s approach to reducing HIV infections in the United States. Accessed September 19, 2017.
- HIV incidence: Estimated annual infections in the U.S., 2008-2014 [fact sheet]. Accessed September 19, 2017.
- HIV infection risk, prevention, and testing behaviors among men who have sex with men
National HIV Behavioral Surveillance, 20 U.S. cities, 2014. HIV Surveillance Special Report 2016;15. - HIV prevention modeling study [press release]. Accessed September 19, 2017.
- HIV testing experience before HIV diagnosis among men who have sex with men—21 Jurisdictions, United States, 2007–2013. MMWR2016;65(37):999-1003.
- Lifetime risk of HIV diagnosis [press release]. Accessed September 19, 2017.
- Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 201 HIV Surveillance Supplemental Report 2017;22(2).
- New HIV infections drop 18 percent in six years [press release]. Accessed September 19, 2017.
- Trends in U.S. HIV diagnoses, 2005-2014 [fact sheet]. Accessed September 19, 2017.
- Kwan CK, Rose CE, Brooks JT, Marks G, Sionean C. HIV testing among men at risk for acquiring HIV infection before and after the 2006 CDC recommendations. Public Health Rep 2016;131:311-9. PubMed abstract.
- Purcell D, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J 2012;6(Suppl 1: M6):98-107.
- Wejnert C, Le B, Rose CE, et al. HIV infection and awareness among men who have sex with men–20 Cities, United States, 2008 and 2011. PLoS One 2013;8(10).
- https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-hssr-nhbs-msm-2014.pdf
Usa, in aumento i gay che praticano sesso non protetto. Dilagano i party “bareback”
Il dato emerge da un rapporto dei “Centers for Disease Control and Prevention”. Dal 2005 sta aumentando la percentuale di omosessuali maschi che ha avuto rapporti senza preservativo – almeno una volta all’anno – è aumentata del 20%. Un terzo degli uomini intervistati ammette di non aver fatto il test per l’Hiv nell’ultimo anno.
La questione Aids torna quindi prepotentemente a segnare la comunità gay americana.
http://www.tandfonline.com/doi/abs/10.1080/09540120500521137
Il fenomeno è reso ancora più grave dalla frequente associazione con sostanze stupefacenti (cosiddetto sesso chimico) in particolare meta anfetamine, che diminuiscono ulteriormente la prudenza annullando la percezione del rischio.
http://www.tandfonline.com/doi/abs/10.1080/15332980902831114
Sifilide
A questo link ufficiale del WHO (World Health Organization = Organizzazione Mondiale della Sanità, OMS)
http://www.who.int/gho/sti/sex_msm/en/
si possono trovare le ricerche dell’Organizzazione Mondiale della Sanità sulla prevalenza della sifilide nella popolazione omosessuale, che infetta una percentuale di MSM che oscilla tra il 5 e il 20 % a seconda delle varie regioni.
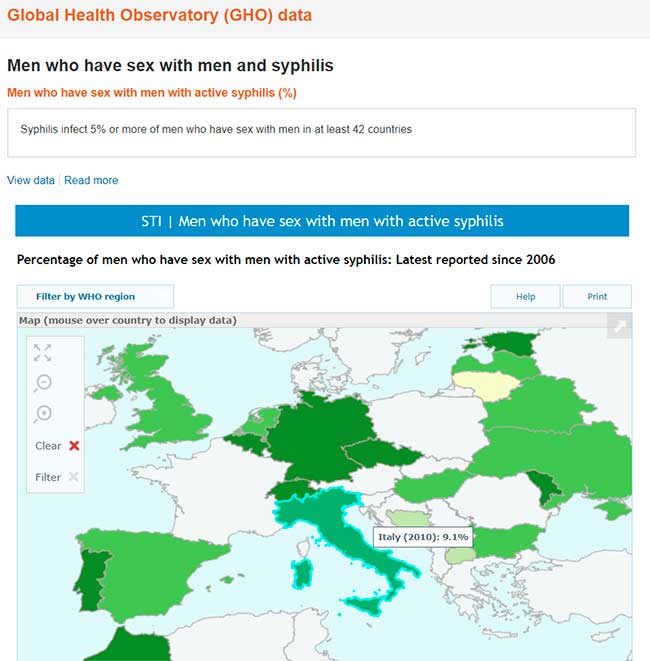
Global Health Observatory (GHO) data Men who have sex with men and syphilis Men who have sex with men with active syphilis (%). Worldwide, syphilis is a highly prevalent infection among men who have sex with men (MSM). Syphilis infects 5% or more of MSM in at least 42 countries, 10% or more in 18 countries, and over 20% in 5 countries. There have been reports from some countries of increasing trends. Untreated syphilis can lead to serious complications in 25% of infected individuals. The complications can be severe and even life-threatening, and increase risk of HIV acquisition and transmission.
Dati di Global Health Observatory (GHO) Uomini che fanno sesso con uomini e sifilide Uomini che fanno sesso con uomini con sifilide attiva (%). In tutto il mondo, la sifilide è un’infezione molto diffusa tra gli uomini che fanno sesso con uomini (MSM). La sifilide infetta il 5% o più di MSM in almeno 42 paesi, il 10% o più in 18 paesi e oltre il 20% in 5 paesi. Ci sono state segnalazioni da alcuni paesi di tendenze in aumento. La sifilide non curata può portare a gravi complicanze nel 25% dei soggetti infetti. Le complicanze possono essere gravi e persino pericolose per la vita e aumentare il rischio di acquisizione e trasmissione dell’HIV.
Dati sovrapponibili in Europa:
Gonorrea
Anche la gonorrea ha un’incidenza nettamente più alta nei MSM. In questi pazienti particolarmente temibile l’associazione con la clamidia. Sempre più preoccupanti le possibilità di trasmissione attraverso la saliva e la antibiotico resistenza. La percentuale varia a seconda delle statistiche e della nazione di rilevamento. Il Rapporto ECDC riferisce infatti una trasmissione dovuta nel 49% dei casi a rapporti etero contro un passaggio omosessuale nel 44% degli episodi segnalati.
- Neisseria gonorrhoeae antimicrobial resistance among men who have sex with men and men who have sex exclusively with women: the Gonococcal Isolate Surveillance Project, 2005-2010. [Ann Intern Med. 2013] Kirkcaldy RD, Zaidi A, Hook EW 3rd, Holmes KK, Soge O, del Rio C, Hall G, Papp J, Bolan G, Weinstock HS. Ann Intern Med. 2013 Mar 5; 158(5 Pt 1):321-8.
- Neisseria gonorrhoeae Antimicrobial Susceptibility Surveillance – The Gonococcal Isolate Surveillance Project, 27 Sites, United States, 2014.[MMWR Surveill Summ. 2016]
- A Cross Sectional Analysis of Gonococcal and Chlamydial Infections among Men-Who-Have-Sex-with-Men in Cape Town, South Africa.[PLoS One. 2015]
- Screening for Asymptomatic Extragenital Gonorrhea and Chlamydia in Men Who Have Sex with Men: Significance, Recommendations, and Options for Overcoming Barriers to Testing.[LGBT Health. 2015]
- Extragenital Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae: A Review of the Literature.[Infect Dis Obstet Gynecol. 2016]
- Duration of gonorrhoea and chlamydia infection at the pharynx and rectum among men who have sex with men: a systematic review.[Sex Health. 2016]
Amebiasi
Una rara malattia parassitaria, che normalmente è trasmessa solo dall’acqua contaminata, ha dimostrato di poter essere trasmessa con l’atto sessuale non protetto tra uomini gay HIV-positivi.
- http://www.antimicrobe.org/new/b137.asp
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2254204/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2600324/
- https://academic.oup.com/cid/article/49/3/346/497179
- Outbreak of intestinal amoebiasis among men who have sex with men, Barcelona (Spain), October 2016 and January 2017 [Eurosurveillance. 2017]Escolà-Vergé L, Arando M, Vall M, Rovira R, Espasa M, Sulleiro E, Armengol P, Zarzuela F, Barberá MJ. Eurosurveillance. 2017 Jul 27; 22(30): 30581
- Prevalent and Incident HIV Diagnoses among Entamoeba histolytica-Infected Adult Males: A Changing Epidemiology Associated with Sexual Transmission — Taiwan, 2006–2013 [PLoS Neglected Tropical Diseas…]
Giardiasi
Una parassitosi intestinale, trasmissibile anche in rapporti che interessino l’ano (oro/anali) o per contaminazione che interessa con maggiore percentuale i MSM: Particolarmente grave nell’associazione con HIV
- http://www.sciencedirect.com/science/article/pii/S0001706X14000035
- http://www.ajtmh.org/content/journals/10.4269/ajtmh.2007.76.549
- http://www.jpmh.org/index.php/jpmh/article/view/344
Herpex simplex anorettale.
In questo studio: Herpes: More Gay Men are infected with the Virus – http://vir123.com/herpes-more-gay-men-are-infected-with-the-virus/ si evidenzia la maggiore presenza di Herpes nei MSM, e, inoltre, una diversa incidenza dal punto di vista etnico:
It also showed that there is a large racial disparity in those infection rates. As Dr. Okafor and colleagues wrote, “In the US, non-Hispanic blacks have the highest life-time seroprevalence (41.8%) of HSV-2 with prevalence nearly four times that of non-Hispanic whites (11.3%).
(1) Brown, E. “Consequences of genital herpes simplex virus infection among vulnerable populations” – Researchgate.net
(2) Brito MO1, Hodge D1, Donastorg Y2, Khosla S3, Lerebours L4, Pope Z3. “Risk behaviours and prevalence of sexually transmitted infections and HIV in a group of Dominican gay men, other men who have sex with men and transgender women.” BMJ Open. 2015 Apr 29;5(4):e007747.
(3) Okafor N1, Rosenberg ES1, Luisi N1, Sanchez T1, Del Rio C2, Sullivan PS1, Kelley CF3. “Disparities in herpes simplex virus type 2 infection between black and white men who have sex with men in Atlanta, GA.” Int J STD AIDS. 2015 Sep;26(10):740-5.
(4) Polansky, H. Itzkovitz, E. Gene-Eden-VIR Is Antiviral: Results of a Post Marketing Clinical Study. Published in September 2013.
(5) CDC.gov – CDC Fact Sheet: What Gay, Bisexual and Other Men Who Have Sex with Men Need to Know About Sexually Transmitted Diseases
Epatite A
Si tratta di un virus orofecale, in netto aumento nella popolazione MSM, motivo per cui è raccomandata la vaccinazione.
Epatite:
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/13-12-2016-RRA-Hepatitis%20A-United%20Kingdom.pdf
- https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/16-02-2017-RRA%20UPDATE%201-Hepatitis%20A-United%20Kingdom.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/RRA-19-May-2017_UPDATE_2-HepatitisA-in-mostly-MSM.pdf
- https://ecdc.europa.eu/sites/portal/files/documents/RRA%20hep%20A%20outbreak%20EU%20EEA%20in%20MSM%20third%20update%2028%20June%202017_0.pdf
Allegato A
http://sti.bmj.com/content/80/4/255.
Recent trends in the epidemiology of sexually transmitted infections in the European Union
Abstract
Sexually transmitted infections (STIs) are a major public health problem in Europe. We review recent trends in the epidemiology of the major acute STIs in the European Union and Norway, their key determinants, and opportunities for enhancing STI prevention interventions in the region.
http://dx.doi.org/10.1136/sti.2004.009415
Sexually transmitted infections (STIs) are a major public health problem in Europe. Their substantial morbidity, associated mortality, and disproportionate burden upon women, marginalised communities, and those with high risk sexual lifestyles1 continue to drive their prioritisation in European public health and policy arenas.2 In many European Union (EU) countries, widescale behavioural modification in response to the emerging HIV/AIDS pandemic saw dramatic reductions in the incidence of many acute STIs in the late 1980s.3–6 However, these have not been maintained and many states are now observing increases in rates of diagnosed STIs.4 In this article we review recent trends in the epidemiology of the major acute STIs in the EU and Norway, their key determinants, and opportunities for enhancing STI prevention interventions in the region.
METHODS
We undertook a review of the published literature, STI surveillance reports, and ad hoc publications related to the distribution of STIs in the EU and Norway in order to describe recent trends in the epidemiology of major STIs in the region. Relevant publications were sought through computerised searches in PubMed from 1980 to 2002. Searches were not restricted by language. Internet searching was undertaken by visiting the websites of the national surveillance centres of all countries in the EU and where possible, collecting information on diagnoses of acute STIs. Where available, we obtained and analysed surveillance data for gonorrhoea, syphilis, and chlamydia for the 15 European countries provided by surveillance leads participating in the European Commission DG SANCO funded European STI Surveillance (ESSTI) Network. We also examined factors influencing the recent increases in STI rates and the contribution of different vulnerable populations to disease transmission. Finally, we accessed the grey literature in this area obtained through a network of STI physicians and epidemiologists. Conference abstracts and other unpublished manuscripts were excluded since the detailed information required was rarely available.
REGIONAL DESCRIPTION: EUROPE—CHANGING DEMOGRAPHY AND IMPLICATIONS FOR STI TRANSMISSION
At the time of writing, the EU comprised 15 member states (table 1), but on 1 May 2004, 10 accession countries (Cyprus, the Czech Republic, Estonia, Hungary, Latvia, Lithuania, Malta, Poland, Slovakia and Slovenia) joined the union. Data in this paper refer to the 15 EU countries and Norway unless otherwise indicated. The EU’s population of 379.0 million increased by 1 290 000 in 2002, an annual rate of 0.3%.7 Net migration accounted for almost three quarters of this rise (table 2). Population increases were largest in Ireland (15.2 per 1000) and Luxembourg (9.5 per 1000) and lowest in Germany (1.2 per 1000) and Italy (1.4 per 1000). Net migration was higher than the natural increase in every member state in 2002, apart from France, Ireland, the Netherlands, and Finland (table 2).
Table 1
Key demographic and migration statistics for selected EU countries (Source: Multiple sources including EUROSTAT.7)
Table 2
Population movements and asylum applications in EU, 2001
The natural increase in the EU has been below net migration since 1989. The declining natural increases in many EU states are largely explained by women having fewer than two children on average coupled with an increasing average age at childbirth.7,8 The total fertility rate in the EU in 2002 remained virtually unchanged at 1.47 children per woman compared with 2001 and 2000. The highest fertility rates are recorded by Ireland (2.01) and the lowest by Greece, Spain (1.25 each), and Italy (1.26). Marriages continue to decline in the EU, falling from 2.2 million in 1980 to 1.8 million in 2002 (−19%). Demographic trends towards earlier coitarche, later childbirth, and declining marriage will have implications for sexual health as they widen the time period available for sex partner acquisition, a key determinant of STI transmission.
Immigration, whether the result of family reunification, economic migration, or asylum seeking, has become an increasingly visible and explosive issue in many western European nations7,9 (tables 1 and 2). The EU is still a major recipient of asylum applications although the number of applications in 2001 fell by 2% compared with 2000 (from 391 460 to 384 530). The main factors influencing people’s migration behaviour include labour market conditions, availability and price of housing, environmental and quality of life considerations, and the effects of government policies. International migration may pose a particular challenge for sexual health as individuals may arrive from, or have contact with, high STI/HIV prevalence countries of origin, thereby increasing their own STI acquisition risk.
INFRASTRUCTURE FOR STI DIAGNOSIS, TREATMENT AND SURVEILLANCE—IMPLICATIONS FOR EU COMPARATIVE STUDIES
There is currently no single, comprehensive source of information on the incidence of, and trends in, diagnosed STIs in the EU. The overall picture has to be assembled from sources that vary in their coverage, detail, and accuracy. In part this reflects the wide variations in STI treatment and care services across the region (see accompanying paper by Lowndes et al10). The United Kingdom is unique in having a dedicated network of treatment centres solely for the management of acute STIs (genitourinary medicine or GUM clinics), from which statistical returns form the basis of national STI surveillance programmes. Data on STIs diagnosed outside of this setting are captured by laboratory reporting or special sentinel surveillance programmes. In contrast, in other EU states, a varying combination of specific STI services, dermato-venereology, public health, and general practice clinics form the basis of STI diagnosis and care, with surveillance data being obtained through voluntary or mandatory reporting from clinics or laboratories; with sentinel or comprehensive coverage.
Lowndes et al10 in a cross sectional survey of EU STI surveillance systems confirm this heterogeneity, whereas comprehensive STI case reporting from dedicated STI treatment centres is the norm in the United Kingdom, and mandatory reporting is required from STI treatment sites Scandinavia, many other EU states rely on a combination of sentinel reporting, mandatory disease notification, or laboratory reporting to monitor disease trends. Considerable variation also exists in the case definitions for the major acute STIs, with some countries requiring laboratory confirmed reports while others require clinical diagnoses or syndromes. Variations in STI screening, partner notification, and treatment practices also influence the degree to which asymptomatic patients and sexual contacts are diagnosed, treated, and recorded in surveillance statistics. Finally, many EU STI surveillance systems are in a state of flux with some countries recently abolishing or developing new national programmes. The net result of this heterogeneity is a severe limitation in our ability to compare disease rates across EU states and in some instances, to monitor disease trends within countries.
DESCRIPTION OF OVERALL TRENDS
However, despite these differences, a number of broad similarities in STI incidence and trends can still be observed across the EU. Most striking is the general reduction in rates of acute bacterial STIs which occurred across the EU throughout the 1980s and early 1990s.3 Gonorrhoea reports fell by between 40–70% in most western EU states between 1991 and 1995, and syphilis reports fell between 10–60% in most states (table 3). These decreases coincided with the emergence of the global HIV/AIDS pandemic, and have been attributed to population-wide behavioural modification in response to HIV campaigns during that time. The disproportionate impact of AIDS related mortality on high risk population subgroups may have also contributed to these decreases.11
Table 3
Recent trends in gonorrhoea and syphilis reports among EU countries
Numbers and rates of acute STIs in many EU countries have however again been on the increase since the mid-1990s. In the United Kingdom, Ireland, and Sweden, diagnoses of gonorrhoea have more than doubled since 1995 and similar magnitude of increases in syphilis reports have been observed in Belgium, the United Kingdom, and Ireland (table 3). Increasing diagnoses of either infection, although of lower magnitude, have been recorded in Spain, Denmark, Austria, France, Finland, Netherlands, and Sweden. The increases have occurred across a range of constituencies but have been most marked among young people, homosexual men, and those resident in major metropolitan areas.
Variations in STI incidence have also been described within some migrant and ethnic minority communities in some EU states, reflecting high disease prevalence in their countries of origin; higher prevalence of risk behaviours; and generally poor access to culturally appropriate STI prevention and treatment services. Finally, many EU states continue to experience evolving commercial sex work networks, partially driven by changing migratory patterns, human trafficking, and illegal drug use. These will continue to offer new opportunities for STI transmission.
DISEASE SPECIFIC TRENDS
Gonorrhoea
A decline in gonorrhoea rates was noted in most western EU countries starting in the 1970s5,12,13 with acceleration in the rates of decline during the second half of the 1980s. Much of this decline continued into the early 1990s. Table 3 summarises gonorrhoea reports from selected EU countries (compiled from national surveillance and WHO databases) between 1990 and 2000, and confirms the near uniform decreases in disease incidence during the first half of this period. By 1995, gonorrhoea rates reach their lowest point in many EU states.
However, EU-wide increases in diagnoses and rates of gonorrhoea have been observed since the late 1990s (table 3). In Belgium, gonorrhoea incidence has increased consistently since 1997. The incidence in men aged 25–44 years rose from 3 per 100 000 population in 1995, to 3.5/100 000 in 1997 and 5/100 000 in 1998.14 In England, Wales, and Northern Ireland gonorrhoea diagnoses more than doubled between 1996 and 2001 with 40% of diagnoses among women occurring in those aged under 20 years. In France, the RENAGO (Réseau National du Gonocoque) network reported an almost twofold increase in the number of patients diagnosed with gonorrhoea between 1997 and 1998, reversing a trend observed since 1987.14 In Ireland there was a 320% increase in notified gonorrhoea cases between 1996 and 2001, with 83 (2.3/100 000) in 1996 and 349 (8.9/100 000) in 2001; 75.9% of cases in 2001 were in men. In Austria, the number of notified gonococcal cases increased from 414 in 2000 to 995 in 2002 with an increase in both men and women. Statutory notification of gonorrhoea began in Spain in 1982 and has fallen continuously since the disease became notifiable. From 1993 to 1999 the rates fell from 18.6 to 3.9 cases per 100 000 population.14
Not all countries have been observing these marked increases. In Norway the number of cases of gonorrhoea reported by laboratories fell steadily from 944 cases in 1990 to 175 in 1995, rose to 223 in 1996, but has remained below 200 since then.14 In Sweden, between 1997 and 2000, increasing number of cases were seen. This trend was reversed in 2001 with a decrease in number of cases, followed by a further decrease in 2002 leading to an infection rate of 5.6/100 000 population. There has however been a noted increase in cases reported in the first half of 2003 compared with the same period in 2002.15,16
In many EU states, gonorrhoea incidence is concentrated among the young, homosexual men, highly sexually active, and socioeconomically deprived communities. In England Wales, and Northern Ireland, 40% of all gonorrhoea diagnoses in women occur in those aged 16–19 years, and 17.4% of all diagnoses in men are acquired through sex between men.17 In Sweden 44.8% of the gonorrhoea cases in 2002 were among men who have sex with men (MSM). In Denmark18 Johansen and Smith confirmed that MSM account for a disproportionately large burden of gonorrhoea in that country with the highest incidence occurring among HIV infected MSM (483.3 per 100 000 population per year) compared to others. There is also a strong association between infection and overseas travel.
Data on gonococcal antimicrobial resistance across the EU is not comprehensive, and this remains an area for future investment. Van Duynhoven5 found that plasmid mediated resistance to penicillin and tetracycline had increased in Europe during the early 1990s. At that time, sporadic resistance to fluoroquinolones had been documented, mainly imported from South East Asia with no resistance to third generation cephalosporins being observed.5 By the mid-1990s, high levels of penicillin positive Neisseria gonorrhoeae (PPNG) were reported in many large metropolitan areas across Europe with prevalences of 13% in Sweden, 6% in Finland, 3% England, 14% in France, and 15–30% in the Netherlands. More recently, increases in flouroquinolone resistance have been reported in many EU states. In Denmark, laboratory confirmed prevalence of fluoroquinolone resistance increased from 0% to 27% in 1999,19 while 17% of strains were resistant to both penicillin and fluoroquinolones.19 In Austria, an increase of the resistance to ofloxacin and ciprofloxacin has been documented from 0% and 3.1%, respectively, in 1999, to 52.3% and 48.8%, respectively, in 2002. Data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales also confirm recent increases in ciprofloxacin resistance. In 2002, 2204 gonococcal isolates were tested, and the overall prevalence of ciprofloxacin resistance (minimum inhibitory concentration > or = 1 mg/l) was 9.8%, compared with 3.1% in 2001 and 2.1% in 2000. Similar increases were reported in Scotland (11% in 2002 compared with 4% in 2001 and 2.8% in 2000). In Sweden a national prevalence study in 1998 tested 348 isolates, representing 89% of diagnosed cases in Sweden, showed 18% of all isolates to have a decreased susceptibility to ciprofloxacin (MIC>0.64 mg/l). This percentage was substantially higher (63%) among individuals who had been exposed in Asia.20
Syphilis
As with gonorrhoea, numbers and rates of infectious syphilis fell to their lowest levels in many EU countries by the early 1990s6(table 3 and fig 1).21 This was despite concomitant and dramatic increases in syphilis incidence in the former USSR.22 The decreases were accompanied by marked reductions in the incidence of congenital syphilis and tertiary disease. In 1995, with the exception of Germany with 1138 reports, fewer than 300 cases of infectious syphilis were reported in any of the other reporting EU countries (table 3). Among these, endemic transmission was rare, with the majority of infections being diagnosed among migrants from high prevalence countries or among EU nationals who had sexual contact outside of the region.
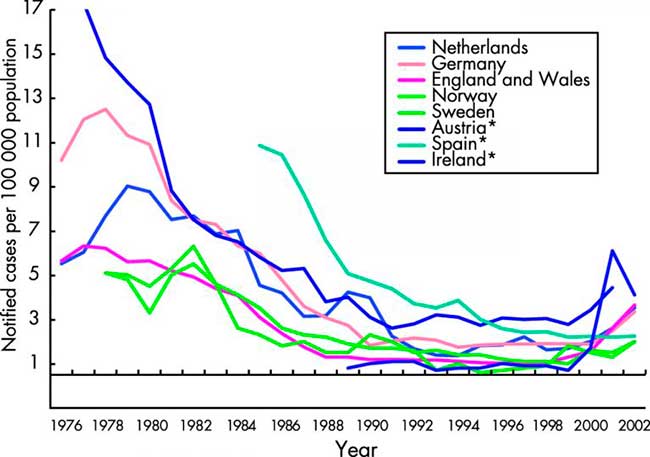
Figure 1
Notifications of infectious syphilis in selected EU countries and Norway. Data from the WHO Computerized Information System for Infectious Diseases (CISID)73and national surveillance databases.31
Since 1996, syphilis has again been on the increase in many northern and western EU states. Several outbreaks of infectious syphilis have arisen in cities across the United Kingdom since the mid-1990s. These have affected homosexual men, heterosexual men and women, newborns, commercial sex workers, and drug users.23,24 In Paris, increases in infectious syphilis cases (30 in 2000 to over 200 in 2002) led to the extension of the Paris syphilis screening campaign to selected French towns and cities targeted at MSM.25 In Belgium, between 2000 and 2002, a 3.5-fold increase in number of laboratory diagnosed syphilis cases was detected by sentinel networks of laboratories. The increases were observed mainly among male patients.26 In Rotterdam, the 56 cases of infectious syphilis (including primary, secondary, early latent) were diagnosed in 2002, compared with 24 cases in 2001 and 16 in 2000.27 In Denmark28 a total of 54 and 51 cases of infectious syphilis were detected during 2000 and 2001, including two cases of congenital syphilis, a substantial increase compared to 1999 when 34 cases were detected. In Finland22 a local cluster or 18 cases was detected in the city of Tampere, the majority of whom were associated with travel to Russia, Estonia, or elsewhere. In Austria, the notified number of syphilis cases steadily increased from 124 in 1993 to 420 in 2002, with about 70% of cases reported in Vienna. In Germany the incidence of syphilis declined significantly throughout the 1990s ranging between 1.2–1.7 per 100 000.29Annual syphilis notifications for the years 1995 to 2000 had been in the range of 1120–1150. In 2001 the syphilis notification system was changed from a mandatory physician based system to laboratory based notification. In 2001, 1681 cases were reported and there were 1102 cases reported in the first 6 months of 2002.30 Much of these increases occurred in large metropolitan German cities.
In Ireland, there has been a dramatic increase in syphilis among MSM in Dublin since early 2000. This was against a low incidence of syphilis nationally throughout the 1990s, which in 1999 reached its lowest level in 10 years (six cases, 0.2/100 000). An enhanced surveillance system for syphilis was set up in 2000, with 48 infectious cases (1.2/100 000) reported in 2000, 219 cases (5.6/100 000) in 2001, and 142 cases (3.6/100 000) in 2002.31 In Norway, a reported outbreak in Oslo among homosexual men (40 cases) contributed to much of the increase in the national incidence rate for 1999, and rates have continued to increase since. In Sweden there was a considerable increase of syphilis cases in 2000 (99 cases) followed by a decrease in 2001, 77 cases of acquired syphilis and one case of congenital syphilis. However, there was an increase in 2002 when 127 cases of acquired syphilis and one case of congenital syphilis were reported; 81% were in men, and among them 71% were in MSM. In 2002, 44% of the infections were acquired abroad, some among asylum seekers and immigrants from high prevalence areas, but also among men involved in homosexual activity abroad. The increase also continued in the first half of 2003 when 85 cases were reported, an increase of 107% compared with the same period in 2002.
Among homosexual men, the resurgence of syphilis has been associated with increasing high risk sexual behaviour, novel sexual networks, use of recreational drugs such as ecstasy and gamma hydroxybutyrate (GHB), sexual activity overseas, and HIV co-infection.32,33 Among heterosexuals risk factors have included sexual activity overseas, migration from high prevalence countries, commercial sex work contact, and drug use. Management of these outbreaks has centred on active partner notification, the establishment of enhanced surveillance of infectious syphilis in the affected areas, and investigation of social and sexual networks facilitating disease transmission. Interventions include enhancing public and healthcare worker awareness of syphilis, venue based screening, rapid testing within and outside of STI treatment centres, targeted sexual health promotion, and syphilis screening of HIV positive individuals.
Genital chlamydial infection
Clinical case notification of genital chlamydial infection is not mandatory in the majority of EU countries and therefore relatively little information is available from national surveillance sources. In the EU countries collecting this information, genital chlamydial infection is now among the most commonly diagnosed bacterial STIs and broadly increasing trends in diagnoses have been observed since the mid-1990s (see fig 2). The surveillance data also confirm the disproportionate disease burden occurring in young women aged less than 20 years. The increasing notification rates are however confounded by concomitant increases in screening rates for this infection and also the increasing use of the highly sensitive nucleic acid amplification tests (NAATs).
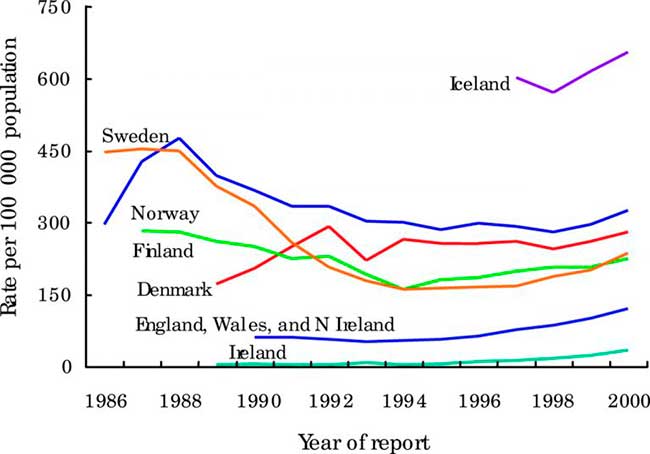
Figure 2
Trends in rates of genital chlamydial infection in selected EU countries and Norway. Data from the WHO Computerized Information System for Infectious Diseases (CISID)73 and national surveillance databases.31
In Denmark, approximately 13 000–14 000 cases of laboratory confirmed genital chlamydia were reported annually during the past decade. By 1999, the highest age specific incidence occurred in the 20–24 year age group among men (950 per 100 000) and in women (2477 per 100 000).19 By 2001, 15 150 cases of chlamydia were diagnosed—an annual incidence of 283 per 100 000. In Ireland, from 1989 to 1995 the number of notified cases of chlamydia generally remained stable, fluctuating around 200 cases per year. In 1995 there was a marked increase of 84.2% on the previous year (from 133 case, 3.7/100 000 to 245 cases, 6.8/100 000). Since then there has been an increasing number of cases reported each year reaching 1649 in 2001. Between 2000 and 2001 the number of male and female cases increased by 15.6% and 28.4% respectively.
In Norway, cases of chlamydia (laboratory notifications) showed a slight decrease from 1986–98 with there being around 12 500 cases in 1998. The peak of reported cases was in 1988 with nearly 20 000 cases being reported. In Sweden, there was a decrease in cases from approximately 350/100 000 inhabitants in 1989 to 160/100 000 each year from 1993 to 1997. Since 1997 there was an increase in cases. In 2002 there were 276/100 000, this represents the same level of infection as 10 years ago and is an 11% increase on 2001. In England, Wales, and Northern Ireland, the number of uncomplicated chlamydia diagnoses in GUM clinics has risen steadily since the mid-1990s and rose by 14% (71 909 to 81 680) between 2001and 2002, a rise of 13% in females and 15% in males. In 2002, the highest rates of chlamydia were among 16–19 year old females (1201/100 000) and 20–24 year old males (837/100 000). Rates in both males and females were highest in the London region (272 and 271/100 000 respectively). In Scotland, laboratory reported cases of chlamydia increased by 17% (10 636 to 12392) between 2001 and 2002—the increase was 16% in males and 20% in females.31 In a large STI diagnostic centre in Vienna the number of chlamydia cases among about 25 000 men and women annually tested increased slightly from 2.3% in 1995 to 3.4% in 2002.31
Because of frequently asymptomatic nature of infections, our understanding of chlamydial epidemiology in Europe is also greatly enhanced by data from ad hoc prevalence studies. Wilson et al,34 in a systematic review of chlamydia prevalence in asymptomatic women, found that the prevalence of C trachomatis in unscreened asymptomatic women in Europe ranging from 1.7% to 17% depending upon the setting, context and country (fig 2).34 The mode was 6% for women seeking contraception and 4% for women having cervical smears. In Vienna, asymptomatic urethral chlamydia infections were observed in young male military recruits in 4.1% when testing urine using nucleic acid amplification techniques.35
Genital herpes
In general, viral STIs are not notifiable in most EU countries10 and relatively few surveillance data are available published on viral STIs in the EU. Where available, increasing trends, albeit at lower rates compared to bacterial STIs, have been described. Genital herpes simplex virus (HSV) infection is the most common ulcerative sexually transmitted infection (STI) in the United Kingdom. Between 1972 and 2002, the number of genital HSV diagnoses made at GUM clinics increased twofold and ninefold in males and females, respectively. The number of diagnoses stabilised and fell briefly in the mid-1980s possibly because of changes in sexual behaviour following extensive media coverage of AIDS. In 2002, 18 392 new cases were reported in England, Wales, and Northern Ireland. For females and males, highest rates were seen in the 20–24 year olds; 2053 per 100 000 population and 93 per 100 000 respectively. In Ireland in 2001, notified cases of genital herpes simplex virus increased by 23.0% on the previous year. HSV cases increased from 78 (2.2/100 000) in 1989 to 198 (5.5/100 000) in 1995 and 331 (8.5/100 000) in 2001. Between 1995 and 2001, the highest rates per 100 000 population were consistently in females and where age group was known, in 20–29 year olds.
In many European countries, seroepidemiological studies provide some insight into the cumulative incidence and prevalence of HSV infection. They confirm the strong association between HSV-2 seroprevalence and increasing age, number of partners during the previous year, as well as the increasing proportion of first episodes of genital herpes being caused by HSV-1 rather than HSV-2. This is especially so among female patients and in the younger age groups with primary or initial disease, where HSV-1 may be the causative viral type in up to 70–90% of cases. The documented change from HSV-2 towards HSV-1 in cases of genital HSV infection may have implications as to prognosis, usefulness of vaccines, and usefulness of new type specific serological tests.
Smith and Robinson36 reviewed published type specific HSV seroepidemiological surveys and found striking variations in HSV-2 prevalence in western Europe. They found HSV-2 prevalence to be higher in northern Europe and North America than in western and southern Europe. The highest prevalence of HSV-2 infection was found among women in Greenland: 57% among those aged 20–24 years and rising to 74% in those 25–39 years.37 In Scandinavia, HSV-2 prevalence was relatively higher than in other areas of Europe, about 15%–35% among women aged 25–35 years.37–41 Three studies in Finland estimated HSV-2 prevalences of 16%, 26%, and 31% among women with mean ages of 30, 39, and 44 years, respectively,42–44 although the studies differed by population, location, and date. In one German study,45 HSV-2 infection was lower among male and female blood donors and hospital patients but was >20% among men and women ⩾60 years.
In Spain, HSV-2 prevalence appeared low (2%–6%) in a large sample of male and female 14–17 year olds,46 in men and women 15–45 years old in eight different geographic areas,47 and in Madrid women.48 In Italy, age specific HSV-2 prevalence was low (0.1%) in a national sample of young male draftees aged 18–25 years,[49. 50] in patients (median age, 26 years) attending a hepatitis B immunisation centre (1.1%),49,50 and in health professionals (mean age, 30 years; 4.8%). In the United Kingdom, HSV-2 prevalence in the general population is low, with the rate of infection significantly lower than that described for the general population in other areas of northern Europe and the United States. In a national UK sample, HSV-2 prevalence was 4.7% among women aged 20–44 years who were controls in a cervical cancer study.51 In this study and in the remaining UK studies, HSV-2 prevalence was <9% for women and men in all age groups.
Genital warts
The most commonly identified manifestations of genital human papillomavirus (HPV) infection are anogenital warts, condyloma acuminata.52 HPV types 6 and 11, responsible for the majority of visible anogenital warts, are commonly regarded as being of low risk with regard to malignancy, and are estimated to affect 1% of sexually active adults aged between 15 and 49.52 Nevertheless, few EU countries routinely collect surveillance data on genital warts infections. In England, Wales, and Northern Ireland, anogenital warts (including new, recurrent and re-registered cases) are the most common viral STI diagnosed at GUM clinics, comprising 10% (69 417 of 675 170) of all diagnoses in 2002. Highest rates of new cases were found in 20–24 year old males (776/100 000) and in 16–19 year old females (681/100 000). In Ireland, anogenital warts accounted for 41.2% of all STI notifications in 2001. Anogenital warts notifications have increased each year since 1992. In 1989, 505 (14.3/100 000) cases were notified, increasing to 1972 (54.4/100 000) cases in 1995 and 3993 cases (101.9/100 000) in 2001. In 2001, 48.8% (1947) of cases were male and 51.2% (2044) were female.
Little is known about the prevalence of HPV in the general population. Routine surveillance data and prevalence studies measuring clinical manifestations of HPV (warts, dysplasia, malignancy) will miss asymptomatic and subclinical infections of the genital tract.53Overall HPV prevalence estimates are also dependent on the age of the sample, the population being sampled, methods used to take clinical specimens, and the polymerase chain reaction (PCR) amplification techniques employed (in particular, which primers are included).54 Whereas many studies report declining HPV prevalence with age,55–58 others have found no age related trend in HPV prevalence or even an indication of an opposite trend.59,60 A population based cohort study carried out in Sweden in 1989 found a baseline HPV prevalence of 22% among sexually active women aged 19–25 (PCR techniques used).59 Studies of women attending routine cervical screening in the United Kingdom in the 1990s have estimated prevalence of HPV “high risk” types of around 6–7%.56,61
Other studies have tended to survey convenience samples of women, usually comprising or including women undergoing gynaecological examination, and thus likely to over-represent women at higher risk.62 A cross sectional study of young Finnish army conscripts (mean age 20) carried out in 1992 found 26% to have any evidence of penile HPV infection (PCR techniques used), although study participants were biased in reporting more sexual risk behaviours or GU symptoms than non-participants.63 Similar prevalence estimates were obtained in a study of Swedish army conscripts (18–23 years), 24% of whom were HPV positive using a variety of detection methods (dot/Southern blot hybridisation and PCR).64 As only half of the conscripts approached agreed to participate, this prevalence may not be reflective of the entire group. A study carried out in Germany in the mid-1980s tested penile swabs obtained from blood donors without signs of genital disease using filter in situ hybridisation techniques and found an overall HPV prevalence of 5.1% in those aged 16–35, and 1.4% prevalence of types 16/18.65 Both the selection criteria and the testing methods applied are likely to make this last study underestimate the prevalence of penile HPV infection in the male population.
DRIVING FACTORS
The consistency of trends across the EU would suggest that, at the macro level, major societal and behavioural determinants of STI transmission are operating in tandem. Those related to the changing demographic, social, and economic structure of the EU (decreases in marriage, delayed childbirth, population movement) have been mentioned previously and will continue to be major influences in the future. Sexual behaviour is, nevertheless, the key determinant of STI transmission and EU-wide changes in the patterns and distribution of high risk sexual behaviour are undoubtedly contributing to the changing disease epidemiology. In many EU countries, the age at first intercourse has stabilised or continues to decline. Data from the recently completed second British National Survey of Sexual Attitudes and Lifestyles (Natsal 2000) confirm the widespread increases in rates of partner acquisition, inconsistent condom use; and homosexual sexual behaviour in the British population over the past decade.66,67Targeted behavioural surveillance surveys (BSS) have also confirmed worsening high risk behaviour among homosexual men in some EU states, with rising trends in reported unprotected anal intercourse being observed in both HIV negative and positive MSM.8,68,69 Similar BSS within young people, migrant communities, and HIV positive individuals will be needed to better interpret disease trends in these groups.
Technological developments are also improving our ability to diagnose and report STIs across the EU. The increasing availability of highly sensitive and specific NAATs, rapid diagnostic tests, and near patient testing for bacterial STIs are improving the ascertainment of infections such as genital chlamydia. Simultaneously, changes in STI screening and diagnostic testing outside of traditional settings are now being advocated in many EU countries and will also contribute to the increasing trend.
Surveillance developments, such as the new sentinel surveillance systems being introduced in Germany, Netherlands, Spain, and Portugal will continue to improve ascertainment and increase STI reports. A review of STI surveillance in Ireland, which is currently under way, together with imminent legislative change and the planned introduction of a national computerised infectious disease reporting (CIDR) system, will greatly improve the quality of the available STI surveillance data.
OPPORTUNITIES FOR SEXUAL HEALTH GAIN
Given the current changes in disease epidemiology, it is clear that considerable health gain is to be obtained from greater investment in effective STI treatment, diagnosis, prevention, and surveillance in all EU countries. At the country level, this must begin by increasing political commitment to sexual health and with the provision of strategic leadership at the highest level. In England, the recent publication of a national sexual heath and HIV strategy,70,71 the appointment of an Independent advisory committee on sexual health, and parliamentary enquiries into sexual health services and migration and HIV72 have served to raise sexual health on the political and public agenda. Nevertheless, much remains to be done. Similar strategies are now being developed in Scotland, Wales, Norway, and Sweden.
Continued investment in STI (including HIV) prevention, microbiological, and surveillance research is also required. Specifically, recent reviews of effectiveness of HIV and STI prevention should be used to guide and prioritise investment in this area. Gains are also likely to be achieved through harnessing the collective expertise and promoting collaboration between laboratory, surveillance, clinical and public health colleagues. The evidence suggests that priority should be given to developing and refining surveillance programmes which are integrated, comprehensive, timely, and responsive to emerging public health priorities10; providing specialist and reference microbiological services for STI agents, and ensuring novel and robust diagnostic technologies are evaluated and applied in cost effective and evidence based manner to improve disease prevention and control activities. Finally, relationships with key external stakeholders, especially those working in HIV/STI prevention and control, should be prioritised.
CONCLUSIONS
Despite the substantial heterogeneity in diagnosis, treatment, and surveillance systems across the EU, available data indicate that STIs are again on the rise in many EU countries. Gains in STI control may be achieved though greater collaboration at the EU level—specifically through harmonising laboratory diagnostic methods, clinical management of STIs, and sharing innovation in STI prevention interventions across the EU. In addition, the heterogeneity of national STI surveillance systems points to the need for more coordinated approaches to EU-wide surveillance, specifically aimed at agreeing case definitions and developing minimum standards for collecting and disseminating STI surveillance data.10 EU-wide coordination is also required in order to confidently interpret EU-wide disease trends. Increasing attention at the EU level will also be required for the identification and management of STI outbreaks.
Acknowledgments
Financial support for this study was provided by the European Commission (DG SANCO), Agreement No SI2.325878 (2001CVG4–018): ESSTI—European Surveillance of Sexually Transmitted Infections.
REFERENCES
- ↵Holmes KK, Sparling PF, Mardh PA, et al.Sexually transmitted diseases. 3rd ed. USA: McGraw-Hill, 1999.
- ↵Wasserheit J, Aral S. The dynamic topology of sexually transmitted disease epidemics: implication for prevention strategies. J Infect Dis1996;174:201–13.
- ↵Van der Heyden JH, Catchpole MA, Paget WJ, Stroobant A, European Study Group. Trends in gonorrhoea in nine western European countries, 1991–6. Sex Transm Infect2000;76:110–6.
Abstract/FREE Full TextGoogle Scholar
- ↵Adler M, Meheust A. Epidemiology of sexually transmitted infections and human immunodeficiency virus in Europe. J Eur Acad Dermatol Venereol2000;14:370–7.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Van Duynhoven Y. The epidemiology of Neisseria gonorrhoeae in Europe. Microbes Infect1999;1:455–64.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Panchaud C, Singh S, Feivelson D, et al. Sexually transmitted diseases among adolescents in developed countries. Fam Plann Perspect2000;32:24–32, 45.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Eurostat. Theme: general statistics. Free data. Available at europa.eu.int/comm/eurostat/, last accessed on 15 January 2004. Eurostat 2004.
- ↵Stolte G, Dukers NH, de Wit JB, et al. A summary report from Amsterdam: increase in sexually transmitted diseases and risky sexual behaviour among homosexual men in relation to the introduction of new anti-HIV drugs. Euro Surveill2002;7:19–22.
- ↵European Commission. The social situation in Europe 2003, in brief. Brussels: European Commission, 2003.
- ↵Lowndes CM, Fenton KA, and the European Surveillance of Sexually Transmitted Infections (ESSTI) Network. Surveillance Systems for STIs in the European Union: Facing a changing epidemiology. Sex Transm Infect2004;80:264–71.
Abstract/FREE Full TextGoogle Scholar
- ↵Chesson HW, Dee TS, Aral SO. AIDS mortality may have contributed to the decline in syphilis rates in the United States in the 1990s. Sex Transm Dis2003;30:419–24.
PubMedWeb of ScienceGoogle Scholar
- ↵Green MS, Anis E, Gandacu D, et al. The fall and rise of gonorrhoea incidence in Israel: an international phenomenon? Sex Transm Infect2003;79:116–8.
Abstract/FREE Full TextGoogle Scholar
- ↵Hiltunen-Back E, Rostila T, Kautiainen H, et al. Rapid decrease of endemic gonorrhea in Finland. Sex Transm Dis1998;25:181–6.
PubMedWeb of ScienceGoogle Scholar
- ↵De Schrijver K, Fenton K, Kiehl W, et al. European trends in gonorrhoea. Eurosurveillance Weekly2000:4.
- ↵Berglund T, Fredlund H, Giesecke J. Epidemiology of the reemergence of gonorrhea in Sweden. Sex Transm Dis2001;28:111–14.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Berglund T, Fredlund H, Ramstedt K. Reemergence of gonorrhea in Sweden. Sex Transm Dis1999;26:390–1.
PubMedWeb of ScienceGoogle Scholar
- ↵Health Protection Agency, SCIEH, ISD, National Public Health Service for Wales, CDSC Northern Ireland, and UASSG. Renewing the focus. HIV and other sexually transmitted infections in the United Kingdom in 2002. London: Health Protection Agency, 1 November 2003.
- ↵Johansen JD, Smith E. Gonorrhoea in Denmark: high incidence among HIV-infected men who have sex with men. Acta Derm Venereol2002;82:365–8.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Hoffmann S. The laboratory surveillance system of Chlamydia trachomatis and Neisseria gonorrhoeae infections in Denmark. Euro Surveill2001;6:86–90.
- ↵Berglund T, Unemo M, Olcen P, et al. One year of Neisseria gonorrhoeae isolates in Sweden: the prevalence study of antibiotic susceptibility shows relation to the geographic area of exposure. Int J STD AIDS2002;13:109–14.
Abstract/FREE Full TextGoogle Scholar
- ↵Nicoll A, Hamers FF. Are trends in HIV, gonorrhoea, and syphilis worsening in western Europe? BMJ2002;324:1324–7.
- ↵Hiltunen-Back E, Haikala O, Koskela P, et al. Epidemics due to imported syphilis in Finland. Sex Transm Dis2002;29:746–51.
PubMedWeb of ScienceGoogle Scholar
- ↵Doherty L, Fenton KA, Jones J, et al. Syphilis: old problem, new strategy. BMJ2002;325:153–6.
- ↵Parkes R, Renton A, Meheus A, et al. Review of current evidence and comparison of guidelines for effective syphilis treatment in Europe. Int J STD AIDS2004;15:73–88.
- ↵Pritchard L, Hoile E. Paris syphilis screening campaign extended to selected French towns and cities. Eurosurveillance Weekly2002:6.
- ↵Hanquet G. IPH ID team. News on outbreak and infectious diseases. Available at Iph.fgov.be/epidemio/epien/Issue19–25 March 2003.
- ↵Van de Laar MJ, van Veen M, Götz H, et al. Continued transmission of syphilis in Rotterdam, the Netherlands. Eurosurveillance Weekly2003:7.
- ↵Axelsen N, Smith E, Koch-Hansen GH. Syphilis cases increasing in Denmark, 2000-01. Eurosurveillance Weekly2002:6.
- ↵Petzoldt D, Jappe U, Hartmann M, et al. Sexually transmitted diseases in Germany. Int J STD AIDS2002;13:246–53.
Abstract/FREE Full TextGoogle Scholar
- ↵Marcus U, Hamouda O, Kiehl W. Results from laboratory-based reporting of syphilis in Germany, 2001–2002. Eurosurveillance Weekly2002:6.
- ↵ESSTI Country STI Surveillance Lead, personal communication,
- ↵Johnson AM, Fenton KA, Mercer C. Phase specific strategies for the prevention, control, and elimination of sexually transmitted diseases: background country profile, England and Wales. Sex Transm Infect2002;78:i125–i132.
Abstract/FREE Full TextGoogle Scholar
- ↵Fenton K, Nicoll A, Kinghorn G. Resurgence of syphilis in England: time for more radical and nationally co-ordinated approaches. Sex Transm Infect2001;77:309–10.
- ↵Wilson JS, Honey E, Templeton A, et al. A systematic review of the prevalence of Chlamydia trachomatis among European women. Human Reproduction Update2002;8:385–94.
Abstract/FREE Full TextGoogle Scholar
- ↵Stary A, Tomazic-Allen S, Choueiri B, et al. Comparison of DNA amplification methods for the detection of Chlamydia trachomatis in first-void urine from asymptomatic military recruits. Sex Transm Dis1996;23:97–102.
PubMedWeb of ScienceGoogle Scholar
- ↵Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis2002;186 (Suppl 1) :S3–28.
- ↵Kjaer SK, de Villiers EM, Haugaard BJ, et al. Human papillomavirus, herpes simplex virus and cervical cancer incidence in Greenland and Denmark. A population-based cross-sectional study. Int J Cancer1988;41:518–24.
PubMedWeb of ScienceGoogle Scholar
- Eskild A, Jeansson S, Jenum PA. [Antibodies against herpes simplex virus type 2 among pregnant women in Norway]. Tidsskr Nor Laegeforen1999;119:2323–6.
- Olsen AO, Orstavik I, Dillner J, et al.Herpes simplex virus and human papillomavirus in a population-based case-control study of cervical intraepithelial neoplasia grade II-III. APMIS1998;106:417–24.
PubMedWeb of ScienceGoogle Scholar
- Persson K, Mansson A, Jonsson E, et al.Decline of herpes simplex virus type 2 and Chlamydia trachomatis infections from 1970 to 1993 indicated by a similar change in antibody pattern. Scand J Infect Dis1995;27:195–9.
PubMedWeb of ScienceGoogle Scholar
- ↵Forsgren M, Skoog E, Jeansson S, et al. Prevalence of antibodies to herpes simplex virus in pregnant women in Stockholm in 1969, 1983 and 1989: implications for STD epidemiology. Int J STD AIDS1994;5:113–6.
PubMedWeb of ScienceGoogle Scholar
- ↵Arvaja M, Lehtinen M, Koskela P, et al. Serological evaluation of herpes simplex virus type 1 and type 2 infections in pregnancy. Sex Transm Infect1999;75:168–71.
- Lehtinen M, Dillner J, Knekt P, et al.Serologically diagnosed infection with human papillomavirus type 16 and risk for subsequent development of cervical carcinoma: nested case-control study. BMJ1996;312:537–9.
Abstract/FREE Full TextGoogle Scholar
- ↵Hakama M, Lehtinen M, Knekt P, et al. Serum antibodies and subsequent cervical neoplasms: a prospective study with 12 years of follow-up. Am J Epidemiol1993;137:166–70.
Abstract/FREE Full TextGoogle Scholar
- ↵Wutzler P, Doerr HW, Farber I, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations—relevance for the incidence of genital herpes. J Med Virol2000;61:201–7.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Gil A, Gonzalez A, Dal-Re R, et al. Prevalence of antibodies against varicella zoster, herpes simplex (types 1 and 2), hepatitis B and hepatitis A viruses among Spanish adolescents. J Infect1998;36:53–6.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Garcia-Corbeira P, Dal-Re R, Aguilar L, et al. Is sexual transmission an important pattern for herpes simplex type 2 virus seroconversion in the Spanish general population? J Med Virol1999;59:194–7.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵De Ory F, Pachon I, Echevarria JM, et al. Seroepidemiological study of herpes simplex virus in the female population in the autonomous region of Madrid, Spain. Eur J Clin Microbiol Infect Dis1999;18:678–80.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Pasquini P, Mele A, Franco E, et al. Prevalence of herpes simplex virus type 2 antibodies in selected population groups in Italy. Eur J Clin Microbiol Infect Dis1988;7:54–6.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Franco E, Caprilli F, Zaratti L, et al. Prevalence of antibodies to herpes simplex virus type 1 in different population groups in Italy. Eur J Clin Microbiol1987;6:322.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Jha PK, Beral V, Peto J, et al. Antibodies to human papillomavirus and to other genital infectious agents and invasive cervical cancer risk. Lancet1993;341:1116–8.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev1988;10:122–63.
- ↵Roman A, Fife KH. Human papillomaviruses: are we ready to type? Clin Microbiol Rev1989;2:166–90.
Abstract/FREE Full TextGoogle Scholar
- ↵Schiffman MA, Brinton LA, Devesa SS, et al. Cervical cancer. In: Schottenfeld D, Fraumeni Jr J, eds. Cancer epidemiology and prevention1996:1090–116.
- ↵Burk RD, Kelly P, Feldman J, et al. Declining prevalence of cervicovaginal human papillomavirus infection with age is independent of other risk factors. Sex Transm Dis1996;23:333–41.
PubMedWeb of ScienceGoogle Scholar
- ↵Cuzick J, Szarewski A, Terry G, et al. Human papillomavirus testing in primary cervical screening. Lancet1995;345:1533–6.
CrossRefPubMedWeb of ScienceGoogle Scholar
- Bauer HM, Hildesheim A, Schiffman MH, et al.Determinants of genital human papillomavirus infection in low-risk women in Portland, Oregon. Sex Transm Dis1993;20:274–8.
PubMedWeb of ScienceGoogle Scholar
- ↵Figueroa JP, Ward E, Luthi TE, et al. Prevalence of human papillomavirus among STD clinic attenders in Jamaica: association of younger age and increased sexual activity. Sex Transm Dis1995;22:114–8.
PubMedWeb of ScienceGoogle Scholar
- ↵Karlsson R, Jonsson M, Edlund K, et al. Lifetime number of partners as the only independent risk factor for human papillomavirus infection: a population-based study. Sex Transm Dis1995;22:119–27.
PubMedWeb of ScienceGoogle Scholar
- ↵Kenney JW. Risk factors associated with genital HPV infection. Cancer Nurs1996;19:353–9.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Cuzick J, Beverley E, Ho L, et al. HPV testing in primary screening of older women. Br J Cancer1999;81:554–8.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Jamison JH, Kaplan DW, Hamman R, et al. Spectrum of genital human papillomavirus infection in a female adolescent population. Sex Transm Dis1995;22:236–43.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Hippelainen M, Syrjanen S, Koskela H, et al. Prevalence and risk factors of genital human papillomavirus (HPV) infections in healthy males: a study on Finnish conscripts. Sex Transm Dis1993;20:321–8.
PubMedWeb of ScienceGoogle Scholar
- ↵Kataoka A, Claesson U, Hansson BG, et al. Human papillomavirus infection of the male diagnosed by Southern-blot hybridization and polymerase chain reaction: comparison between urethra samples and penile biopsy samples. J Med Virol1991;33:159–64.
PubMedWeb of ScienceGoogle Scholar
- ↵Grussendorf-Conen EI, Meinhof W, de Villiers EM, et al. Occurrence of HPV genomes in penile smears of healthy men. Arch Dermatol Res1987;279:S73–S75.
- ↵Johnson AM, Mercer CH, Erens B, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet2001;358:1835–42.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Wellings K, Nanchahal K, Macdowall W, et al. Sexual behaviour in Britain: early heterosexual experience. Lancet2001;358:1843–50.
CrossRefPubMedWeb of ScienceGoogle Scholar
- ↵Dodds J, Nardone A, Mercey D, et al. Increase in high risk sexual behaviour among homosexual men, London 1996-8: cross sectional, questionnaire study. BMJ2000;320:1510–1.
- ↵Laporte A. A new decline in preventive behaviours among homosexual men: the role of highly active antiretroviral therapy? Euro Surveill2002;7:15–6.
- ↵Department of Health. The national strategy for sexual health and HIV. London: DoH, 2001:1–53.
- ↵Department of Health. The national strategy for sexual health and HIV: implementation action plan. London: DoH, 2002.
- ↵All-Party Parliamentary Group on AIDS. Migration and HIV: improving lives in Britain. July 2003. Available at appg-aids.org.uk/Publications/Migration%20and%20HIV%20Improving%20Lives.pdf.
- ↵World Health Organization. CISID—the Computerized Information System for Infectious Diseases. Geneva: WHO Regional Office for Europe, 2004.
Footnotes
↵* The ESSTI Network: Members of ESSTI Collaborative and Steering Groups
Austria: Reinhild Strauss, BM for Social Security and Generations, Vienna; Angelika Stary, Outpatient Center for Diagnosis of Infectious Venerodermatological Diseases, Vienna. Belgium: Andre Sasse, Epidemiology Section, Scientific Institute of Public Health, Brussels; Marjan Van Esbroeck, Institute of Tropical Medicine, Antwerp. Denmark: Else Smith, Department of Epidemiology, Statens Serum Institut, Copenhagen; Steen Hoffmann*, Department of Epidemiology, Statens Serum Institut, Copenhagen. Finland: Angela Rose, Department of Infectious Disease Epidemiology, National Public Health Institute (KTL), Helsinki; Pentti Huovinen, Antimicrobial Research Laboratory, National Public Health Institute, Turku. France: Veronique Goulet; Françoise Hamers*, Institut de Veille Sanitaire; Dépt des maladies infectieuses, St Maurice; Patrice Sednaoui, Laboratoire de Bactériologie, Institut Fournier, Paris. Germany: Osamah Hamouda, Infektionsepidemiologie/AIDS-Zentrum, Robert Koch Institut, Berlin; Peter Kohl, Department of Dermatology and Venereology, Neukolln Academic Hospital, Free University of Berlin. Greece: Mina Psichogiou, Hellenic Center for Infectious Diseases Control, Department for Surveillance and Intervention, Athens; Eva Tzelepi, National Reference Center for N gonorrhoeae, Hellenic Pasteur Institute, Athens. Ireland: Mary Cronin, National Disease Surveillance Centre, Dublin. Italy: Barbara Suligoi*, Laboratory of Epidemiology and Biostatistics, Istituto Superiore di Sanità, Rome; Paola Stefanelli, Dept of Infectious, Parasitic and Immuno-mediated Diseases, Istituto Superiore di Sanità, Rome. Netherlands:Marita van de Laar*, National Institute of Public Health and the Environment (RIVM), Department of Infectious Disease Epidemiology, Bilthoven; Joke Spaargaren, GG and GD Amsterdam, Public Health Laboratory, Amsterdam. Norway: Preben Aavitsland, Norwegian Institute of Public Health, Oslo; Jorgen Lassen, Norwegian Institute of Public Health, Oslo. Portugal: Jacinta Azevedo, Consulta de DST do Centro de Saude da Lapa, Lisbon; Maria Jose Borrego, Centro de Bacteriologia, Instituto Nacional de Saude Dr Ricardo Jorge, Lisbon. Spain: Jesús Castilla, Centro Nacional de Epidemiología, Instituto de Salud Carlos lll, Madrid; Julio Vazquez, Centro Nacional de Microbiologia, Madrid. Sweden: Torsten Berglund*, Swedish Institute for Infectious Disease Control, Solna; Johan Giesecke*, Swedish Institute for Infectious Disease Control, Solna; Hans Fredlund, Swedish Reference Laboratory for Pathogenic Neisseria, University Hospital, Örebro. UK: Mike Catchpole*, Health Protection Agency Communicable Disease Surveillance Centre, London; Hugh Young*, Scottish Neisseria gonorrhoeae Reference Laboratory (SNGRL), Laboratory Medicine (Microbiology), Edinburgh Royal Infirmary, Edinburgh; Chris Bartlett*, UCL Centre for Infectious Disease Epidemiology, Department of Primary Care and Population Sciences, London; Cathy Ison*, Department of Infectious Diseases and Microbiology, Imperial College School of Medicine, London; Jodi Cooper, Health Protection Agency Communicable Disease Surveillance Centre, London; Anne Scoular, Scottish Centre for infection and Environmental Health, Glasgow.
Member of ESSTI Steering Group.
We recommend:
C M Lowndes et al., Sex Transm Infect
A E Brown et al., Sex Transm Infect
N Macdonald et al., Sex Transm Infect
M Hourihan et al., Sex Transm Infect
M W Adler, Sex Transm Infect
Sami L. Gottlieb et al., Medscape
- Highlights From the 2002 STD Conference
- Hunter Handsfield, MD, Medscape
- Prevalence of Sexually Transmitted Co-infections in HIV/AIDS
Seth C Kalichman et al., Medscape
BMJ-British Medical Journal, ScienceDaily
European Centre for Disease Prevention and Control (ECDC), ScienceDaily